MDSAP: Medical Device Single Audit Program
Looking for an MDSAP PDF certification? The Medical Device Single Audit Program (MDSAP) allows medical device manufacturers to undergo a single regulatory audit that meets the requirements of multiple countries. In this guide, we will explore the key aspects of MDSAP certification, including its significance, steps required for certification, benefits of implementation, and more.

What is the Medical Device Single Audit Program? Copied
The Medical Device Single Audit Program (MDSAP) sets internationally standardized requirements for the quality management systems of medical device manufacturers.
Participating countries and associated regulatory bodies include:
- Therapeutic Goods Administration of Australia
- Brazil’s Agência Nacional de Vigilância Sanitária
- Health Canada
- Japan’s Ministry of Health, Labour and Welfare, and the Japanese Pharmaceuticals and Medical Devices Agency
- U.S. Food and Drug Administration (FDA)
- European Union (EU)
- Singapore’s Health Sciences Authority (HSA)
- United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA)
- The World Health Organization (WHO) Prequalification of In Vitro Diagnostics (IVDs) Programme
- Argentina’s National Administration of Drugs, Foods and Medical Devices (ANMAT)
- Ministry of Health of Israel
- Kenya’s Pharmacy and Poisons Board
- Republic of Korea’s Ministry of Food and Drug Safety
- Federal Commission for Protection from Sanitary Risks (COFEPRIS) of Mexico
- South African Health Products Regulatory Authority (SAHPRA) (NEW)
- Taiwan Food and Drug Administration (TFDA)
Who needs a MDSAP certification? Copied
Medical device manufacturers that want to sell their products in MDSAP-participating countries may need MDSAP (Medical Device Single Audit Program) certification. For example, while the program is voluntary in Australia, Brazil, Japan, and the United States, it is mandatory in Canada.
The program can streamline and simplify regulatory compliance, regardless of whether it is required.
How to earn a MDSAP certification Copied
To earn a MDSAP certification, medical device manufacturers can follow a few general steps. However, depending on the type of organization or device, you may need to slightly adjust your approach. Be sure to consult with the regulatory bodies in your region.
- Choose an Auditing Organization (AO). MDSAP audits are performed by MDSAP-recognized AOs, not government agencies. You must select and contract one. A list of approved AOs is available on the FDA’s website
- Prepare for the audit. Implement a quality nanagement system (QMS) that aligns with ISO 13485 and the specific requirements of MDSAP. You should also review MDSAP audit model documents to understand what will be assessed, as well as conduct an internal audit to identify and fix any skill gaps you discover
- Undergo the MDSAP audit. The AO will perform a Stage 1 (document review) and Stage 2 (on-site) audit. They check compliance with both ISO 13485 and country-specific regulations, such as the FDA’s Quality System Regulations and Health Canada’s Medical Device Regulations
- Receive the audit report and address findings. If non-conformities are found, you must provide corrective actions. Once approved, the AO issues a certification valid for three years, with annual surveillance audits
Renewal requirements for MDSAP certifications Copied
MDSAP certifications are valid for three years. During the first two years, certified organizations are subject to annual surveillance audits. In the third year, they will under a recertification audit.
The benefits of MDSAP certification Copied
A MDSAP certification offer several benefits to employees and their organizations. Some key advantages include the following
Reduced regulatory burden
By replacing multiple country-specific audits with a single audit, organizations can simplify processes – and potentially save time and costs while ensuring consistency and compliance in various markets.
Efficient regulatory oversight
Streamlined regulatory oversight allows manufacturers to efficiently monitor their regulatory compliance processes. For example, they can focus on high-risk areas that ensure patient safety while reducing unnecessary administrative workload.
Better resource allocation
Medical deice manufacturers can direct efforts toward innovation and quality improvement, while regulators can focus on critical compliance issues rather than redundant audit processes.
Challenges posed by the MDSAP Copied
Earning a MDSAP certification may pose several challenges to employees and organizations. They include the following.
Complex compliance regulations
MDSAP integrates multiple regulatory frameworks that can require deep knowledge of country-specific rules. Manufacturers must align their QMS with diverse standards, potentially resulting in increased documentation workload or the need for specialized compliance teams.
Increased audit difficulty
MDSAP audits are more comprehensive than traditional audits performed by a single country. The structured approach MDSAP takes scrutinizes every aspect of quality management, with even minor nonconformities potentially leading to findings that affect market approvals or product timelines.
Higher implementation costs
Small and mid-sized manufacturers may struggle with costs associated with consultant support, internal audits, and maintaining ongoing compliance with evolving regulatory expectations across multiple jurisdictions.
Tips and strategies for earning a MDSAP certification Copied
Here are a few tips for preparing for a MDSAP certification:
- Read or study the FDA’s MDSAP documents
- Familiarize yourself with the countries participating in MDSAP
- Conduct a skill gap analysis of requirements
- Train staff on the MDSAP audit process
- Perform internal audits before certification
Resources for MDSAP certification Copied
For more information and guidance on MDSAP certification, you can refer to the following resources:
International Medical Device Regulators Forum. The IMDRF provides links on its website to each governmental agency and regulatory body that is part of MDSAP. It’s a great source of country-specific resources for organizations seeking MDSAP certification
Governmental agencies. The websites of governmental agencies like the FDA offer a wealth of resources for organizations seeking MDSAP certification. Choose the agency most relevant to your country.
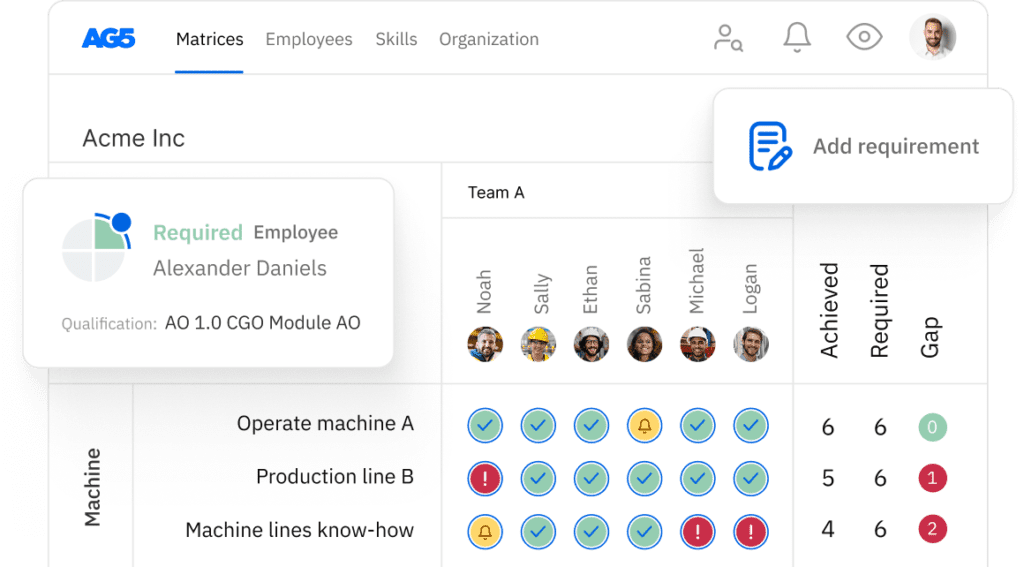
Skills management for MDSAP certifications Copied
AG5 stores all certifications in the cloud, providing all authorized personnel with access to the right version of approved certifications. This helps you easily keep track of all data and documentation related to a MDSAP certifications across your organization.
Using AG5’s skills management software, you can monitor the status of any type of certification that is relevant to your workforce, leveraging intuitive dashboards that provide you with a clear understanding of exactly what is needed to keep your employees skilled and safe.
Frequently asked questions about MDSAP certification Copied
-
What is the scope of MDSAP certification?
-
Is a MDSAP certification mandatory?
-
How long does it take to obtain a MDSAP certification?
-
What are the cost considerations for MDSAP certification?
-
What is the validity period of a MDSAP certification?
-
Can a MDSAP certification be integrated with any management systems?
-
How can you learn more about MDSAP certification?
Sources Copied
Author Copied
Revisions Copied
Tired of managing skills in Excel?
Say goodbye to Excel matrices. Start using AG5’s plug and play skill matrix software.
Recognized by G2 for Excellence in Skills Management